Medication Risk-Benefit Calculator
This tool helps you understand whether the benefits of your medication outweigh the risks based on the FDA's black box warning criteria. Enter your personal health details to get a personalized risk assessment.
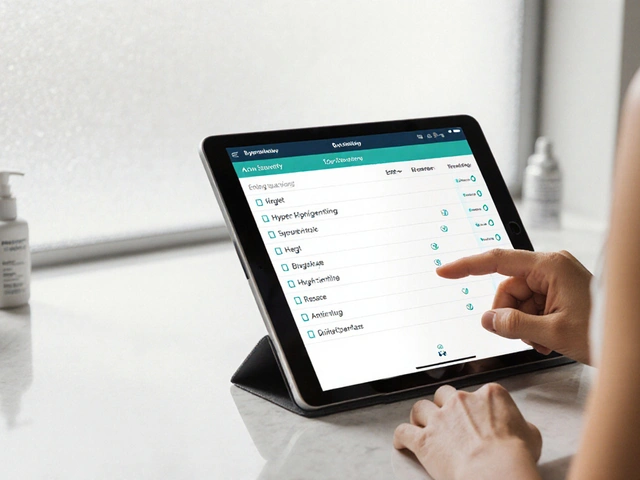
Your Risk-Benefit Assessment
Key Considerations
Safety: Your specific risk level based on black box warning criteria
Tolerability: How side effects impact your daily life
Effectiveness: How well the medication controls your condition
Price: Cost relative to benefits
Simplicity: Ease of correct usage
Recommendation:
When a drug comes with a black box warning, it’s not just a footnote. It’s the FDA’s loudest alarm bell - a clear signal that this medication can cause serious harm, even death, under certain conditions. You’ve probably seen it: a thick black border around bold text at the top of a prescription label. But what does it really mean? And should you be scared if your pill has one?
What Is a Black Box Warning?
A black box warning is the strongest safety alert the U.S. Food and Drug Administration (FDA) can issue. It’s not a suggestion. It’s a requirement. The FDA mandates this warning when evidence shows a drug carries a serious risk of death or severe injury - and that risk is preventable if handled correctly.
The warning appears in a black-bordered box right at the beginning of the drug’s official prescribing information. It’s also included in patient package inserts and even some promotional materials. This isn’t just for doctors. Patients should know about it too. As of 2022, more than 400 prescription drugs carried this warning. That includes medications for diabetes, depression, seizures, pain, and even some antibiotics.
It’s not new. The FDA started requiring these warnings after the Kefauver-Harris Amendments of 1962, which forced drugmakers to prove their products were both safe and effective. But the black box format itself became standard in the 1970s. Today, it’s the gold standard for communicating the gravest risks.
Why Does the FDA Use Black Box Warnings?
The goal is simple: stop preventable harm. These warnings aren’t meant to scare people away from useful drugs. They’re meant to make sure doctors and patients fully understand the danger before using them.
The FDA only adds a black box warning after reviewing solid evidence - often from real-world use, not just clinical trials. Many of these warnings come years after a drug hits the market. That’s because rare but deadly side effects don’t always show up in small trial groups. They appear when thousands of people start taking the drug.
Here are the three main reasons the FDA issues a black box warning:
- The risk is so serious, it might outweigh the benefits for some patients
- The side effect can be reduced with careful use - like regular blood tests or avoiding certain other medications
- Only certain people should take the drug - like those with specific health conditions or age groups
For example, some antidepressants carry a black box warning for increased suicidal thoughts in young adults under 25. That doesn’t mean no one under 25 should take them. It means doctors need to monitor closely during the first few months.
How Is It Different From Other FDA Warnings?
Not all warnings are created equal. The FDA uses several types of safety communications:
- Regular warnings - Mention possible side effects, but they’re common or less severe
- Safety alerts - Temporary notices about emerging risks, often sent to doctors
- Label changes - Updates to the drug’s full prescribing information
- Black box warnings - The highest level. Only for life-threatening or permanently disabling risks
Think of it like a traffic light. A yellow light says “slow down.” A red light says “stop.” The black box warning is the red light. It’s the one thing that forces a pause - a conversation, a check, a second opinion.
One study showed that when the diabetes drug rosiglitazone got a black box warning for heart risks, prescriptions dropped by 70%. But another drug, pioglitazone, with a similar warning, didn’t see the same drop - because it got less media attention. That’s a clue: the warning alone isn’t always enough. Awareness matters.

What Should You Do If Your Medication Has a Black Box Warning?
If you’re taking a drug with a black box warning, don’t panic. But do pay attention.
First, understand the risk. The warning will say exactly what could go wrong. Maybe it’s liver damage. Maybe it’s a rare but deadly allergic reaction. Maybe it’s a risk of birth defects. Know what you’re dealing with.
Second, talk to your doctor. Ask:
- Why am I on this drug instead of another?
- What are the specific signs I should watch for?
- Do I need blood tests or other monitoring?
- Are there safer alternatives?
Some drugs with black box warnings are still essential. For example, certain epilepsy drugs have a warning for severe skin reactions - but for people with uncontrolled seizures, the risk of not taking the drug is far worse.
Doctors are trained to weigh risks and benefits. But they need you to speak up. If you’re worried, say so. If you notice something strange - a rash, unusual fatigue, mood changes - report it. The FDA tracks these reports through its MedWatch system. That’s how they find new dangers.
How Do Doctors Decide Whether to Prescribe a Drug With a Black Box Warning?
It’s not a yes-or-no decision. It’s a conversation.
The American Academy of Family Physicians recommends using the STEPS framework when evaluating these drugs:
- Safety - What are the real risks? Are they avoidable?
- Tolerability - How do side effects affect daily life?
- Effectiveness - Does it actually work better than alternatives?
- Price - Is it worth the cost, especially if monitoring is needed?
- Simplicity - How hard is it to take correctly?
Doctors also check for drug interactions. A black box warning might say “avoid with alcohol” or “don’t take with statins.” Missing that could be dangerous.
They also use independent resources like the Drug Effectiveness Review Project or Consumer Reports Best Buy Drugs. These sites compare drugs based on real-world data - not just marketing.

Are Black Box Warnings Effective?
They work - sometimes. Sometimes they don’t.
The rosiglitazone example shows that when a black box warning gets media attention, prescribing drops sharply. But when it doesn’t, doctors may keep prescribing it, especially if they believe the benefits outweigh the risks.
Experts agree: the warning is a tool, not a cure. It raises awareness, but it doesn’t guarantee behavior change. Some doctors still overlook it. Some patients don’t understand it.
The FDA knows this. That’s why they’re working on better ways to communicate risk - like using numbers instead of vague terms. Instead of saying “serious risk,” they might say “1 in 500 patients may develop liver failure.” That’s clearer. That’s more useful.
What’s Next for Black Box Warnings?
The system isn’t perfect. But it’s still the best tool we have.
Recent updates have focused on clarity. Newer warnings include more specific instructions: “Monitor liver enzymes monthly,” “Avoid in patients with history of heart failure,” “Do not use during pregnancy.”
The FDA is also pushing for more transparency. They now publish the data behind each warning - not just the final text. That means researchers, doctors, and even patients can dig into the evidence.
And patient advocacy groups are getting louder. Some have successfully pushed for black box warnings on drugs that were previously considered safe. That’s a sign the system is responsive - when people speak up, the FDA listens.
Looking ahead, the goal isn’t to eliminate these warnings. It’s to make them more useful. To turn them from a scary box into a clear guide.
Final Thoughts
A black box warning doesn’t mean “never take this.” It means “take this with eyes wide open.”
If you’re on one of these drugs, you’re not alone. Millions are. Many live healthy lives because their doctors understood the risk - and managed it.
But knowledge is power. Don’t assume your doctor knows everything. Don’t assume the label says everything. Ask questions. Do your homework. And if you’re unsure, get a second opinion.
The FDA didn’t create black box warnings to scare you. They created them to protect you. But protection only works if you understand it.
Does a black box warning mean I can’t take the medication?
No. A black box warning doesn’t mean the drug is banned or unsafe for everyone. It means the risks are serious enough that your doctor must carefully weigh them against the benefits. Many people take these medications safely with proper monitoring and guidance.
Can a drug get a black box warning after it’s already on the market?
Yes. In fact, most black box warnings are added after a drug is already being used widely. The FDA monitors real-world use through reports from doctors, patients, and pharmacies. If new, serious side effects emerge - like liver failure or heart problems - they can require a warning update.
How do I find out if my medication has a black box warning?
Check the patient information leaflet that comes with your prescription. It’s always at the top. You can also look up your drug on the FDA’s website or use trusted pharmacy tools like GoodRx or Drugs.com. If you’re unsure, ask your pharmacist.
Should I stop taking my medication if it has a black box warning?
Never stop a prescription medication without talking to your doctor first. Stopping suddenly can be dangerous - even more than the warning itself. If you’re concerned, schedule a visit to discuss alternatives, monitoring, or whether the benefits still outweigh the risks for you.
Can I report a side effect from a drug with a black box warning?
Yes. The FDA encourages reports from patients, doctors, and pharmacists through its MedWatch program. Even if the warning is already in place, new reports help the FDA understand how often side effects happen and whether the warning needs updating. You can report online at fda.gov/medwatch or by calling 1-800-FDA-1088.





Ashley Hutchins
I swear people just ignore these warnings like they're just suggestions. My cousin was on that diabetes drug and didn't get her liver checked once. Now she's on a transplant list. Black box means BLACK BOX. Not 'maybe pay attention'.
Stop being lazy. Your life isn't a gamble.
Lakisha Sarbah
I had a black box warning on my antidepressant and honestly? It scared me at first. But my doctor sat down with me, explained exactly what to watch for, and we set up weekly check-ins. It worked. The warning wasn't there to scare me off-it was there to help me stay safe. Knowledge is power, not panic.
Ariel Edmisten
Don't stop meds. Talk to your doctor. That's it.
Savannah Edwards
I grew up in a family where meds were never questioned. My grandma took five different pills every day and never read the leaflets. When I finally got my first black box warning for an epilepsy drug, I cried. Not because I was scared, but because I realized how much trust we put in the system without ever asking questions. It made me rethink everything-how we talk about health, how doctors assume we know what to do, how we silence our own instincts because 'the doctor said so.' I started keeping a journal. I brought it to every appointment. I asked for alternatives. I found a pharmacist who actually listened. It changed my life. And yeah, I still take the drug. But now I know why. And that matters more than any warning box ever could.
AMIT JINDAL
Lmao Americans and their overregulated meds 😂 I work in pharma in India and we don't have these black boxes. People just take what works. If you get sick? Well, maybe you shouldn't have taken it. 😎 We use drugs based on results, not fear-mongering labels. This FDA thing is just corporate overkill. Plus, who reads all that? I mean, come on. 🤷♂️
Niel Amstrong Stein
Honestly? I think the black box is less about the drug and more about the system not wanting to get sued.
It’s like a legal shield wrapped in bold text.
But I get it. I’ve been on one. Took it for 3 years. No issues. But I tracked my sleep, my moods, my bloodwork. Just because the warning exists doesn’t mean it’s your fate. It just means you’re now an adult in your own healthcare. Welcome to the club. 🤙
Mary Carroll Allen
I read this whole thing and I’m FURIOUS. Why do we wait until someone dies to put a warning on a drug? Why aren’t these things built into the approval process from day one? I have a friend who lost her sister to a reaction that was reported 14 times before the black box came out. FOURTEEN TIMES. And we still don’t have mandatory reporting from pharmacies? This isn’t safety. This is negligence dressed up as regulation. I’m done being polite about this. Someone needs to burn the system down and rebuild it. #FDAFail
Joey Gianvincenzi
The utilization of black box warnings constitutes a regulatory imperative grounded in evidence-based pharmacovigilance. It is incumbent upon the medical community to ensure that informed consent is not merely a procedural formality but a substantive, cognitively engaged dialogue between clinician and patient. The FDA’s mandate in this regard is not merely advisory-it is a foundational pillar of ethical pharmaceutical practice. Failure to adhere to these standards constitutes a breach of the Hippocratic Oath in its most literal sense.
Amit Jain
You people are so scared of medicine. It’s not a horror movie. Black box? Big deal. My cousin took that exact antidepressant and it saved his life. He’s now a pastor. The real danger is letting fear stop you from getting help. You’re all just hypochondriacs with Wi-Fi. Go outside. Touch grass. Stop reading FDA PDFs like they’re sacred scripture. #StopThePanic