When you pick up a prescription, you might not realize that the pill in your hand could be a generic version of a brand-name drug. But here’s the thing: if it’s listed as therapeutic equivalence in the FDA’s Orange Book, it’s not just cheaper-it’s designed to work exactly the same way. For millions of people, this isn’t a theory. It’s daily reality. And it’s one of the quietest, most important safety nets in modern medicine.
What therapeutic equivalence really means
Therapeutic equivalence isn’t just about having the same active ingredient. That’s pharmaceutical equivalence, and it’s only the first step. Two drugs can have the same chemical compound and still behave differently in your body. Therapeutic equivalence goes further. It means two drugs-whether brand or generic-produce the same clinical effect and carry the same safety profile when taken under the same conditions. The U.S. Food and Drug Administration (FDA) sets this standard. They don’t just assume it. They test it. And they don’t cut corners. To earn a therapeutic equivalence rating, a generic drug must pass two strict tests: bioequivalence and clinical outcome consistency. Bioequivalence means the generic drug gets into your bloodstream at the same rate and in the same amount as the brand-name version. The FDA requires the 90% confidence interval for the drug’s absorption (measured by AUC and Cmax) to fall between 80% and 125% of the original. That’s not a guess. It’s math. It’s data. It’s repeated across dozens of healthy volunteers in controlled studies. And then there’s the clinical part. The FDA doesn’t just look at blood levels. They look at what happens in real patients. Does the generic control seizures like the brand? Does it lower blood pressure just as reliably? If the answer is yes across multiple studies, it gets an ‘A’ rating in the Orange Book. That’s the green light for substitution.How the FDA’s Orange Book keeps patients safe
The Orange Book isn’t just a list. It’s a living database that tracks over 13,000 drug products as of 2023. Each entry has a two-letter code. ‘AB’ means the generic is therapeutically equivalent to the brand. ‘B’ means there’s a known or suspected issue-maybe the formulation is too complex, or bioequivalence couldn’t be proven. This system matters because not all drugs are created equal. For drugs like warfarin, levothyroxine, or phenytoin-those with a narrow therapeutic index-there’s almost no room for error. A 5% difference in absorption could mean a blood clot or a seizure. That’s why the FDA applies stricter standards for these: 90-110% bioequivalence instead of 80-125%. They know the stakes. Pharmacists rely on this code every day. In 49 states, they can legally swap a brand drug for a generic with an ‘AB’ rating without asking the doctor. That’s because the FDA has already done the work. The system exists to protect patients-not to save money, though that’s a side effect. It’s about making sure the medicine you get today works the same as the one you got last month.Why therapeutic equivalence beats therapeutic interchange
Some people think swapping one drug for another in the same class-say, switching from one statin to another-is the same as therapeutic equivalence. It’s not. Therapeutic interchange means replacing a drug with a different active ingredient that treats the same condition. That’s not the same as replacing a brand with its generic twin. A 2019 study in JAMA Internal Medicine found that therapeutic interchange led to 32% more adverse events than substitution based on therapeutic equivalence. Why? Because different drugs have different side effects, interactions, and absorption patterns. Even small differences matter. Therapeutic equivalence, by contrast, keeps the active ingredient the same. You’re not changing the drug-you’re changing the manufacturer. And that’s why it’s so safe.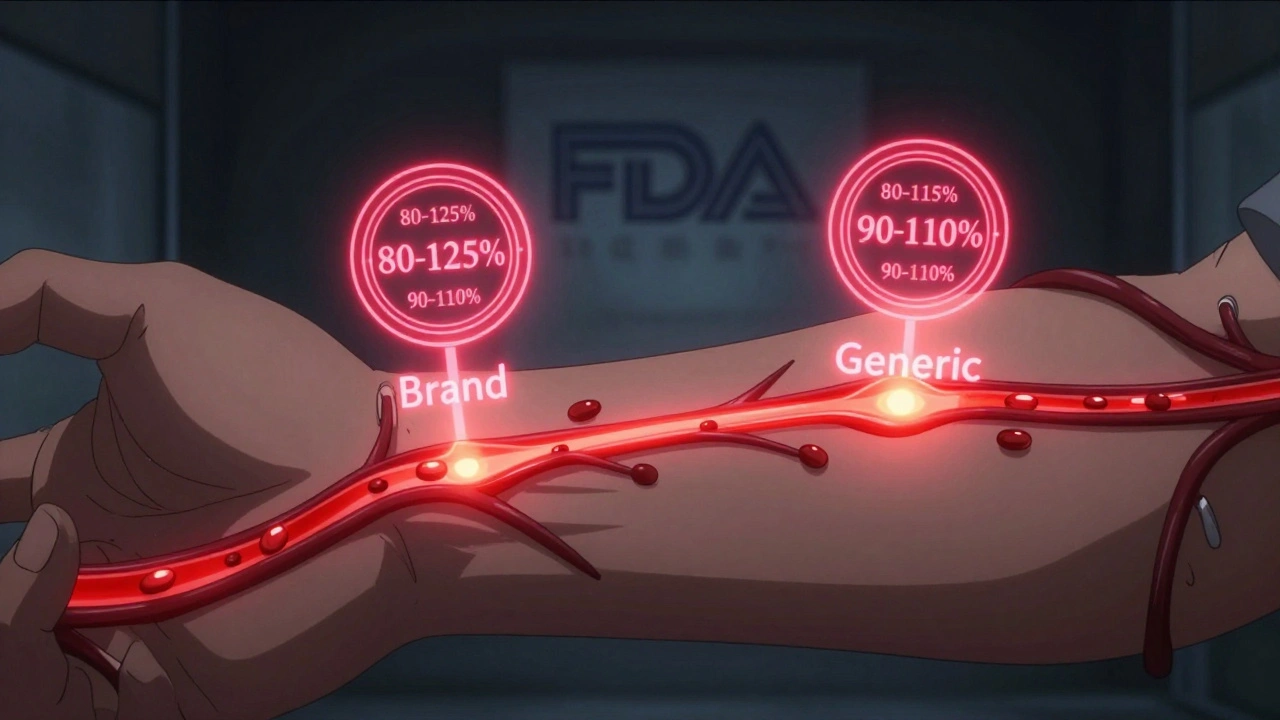
What about the scary stories?
You’ve probably heard them. Someone switched to a generic and felt worse. Their blood pressure spiked. Their thyroid levels went haywire. They blamed the generic. It’s understandable. Change is unsettling. But when you dig into the data, the picture changes. A 2022 survey of 12,500 UnitedHealthcare patients found 87% reported no difference in how the generic worked. Only 3.2% linked any side effects to the switch. The Institute for Safe Medication Practices reviewed 127 reports of adverse events tied to generic substitution between 2018 and 2022. Only 17 involved drugs with an ‘AB’ rating. The rest? Either drugs with ‘B’ ratings (already flagged as potentially unsafe), or cases where patients were switched to a different drug entirely-not a true generic. Even Reddit threads about generic problems often turn out to be misattributed. One popular thread from 2021 listed 47 cases. Only three involved an ‘AB’-rated product. The rest were mix-ups, anxiety, or unrelated health changes. The truth? Most people don’t notice a difference. And when they do, it’s rarely because the drug didn’t work. It’s because they expected it to.Complex drugs and the next frontier
Not all drugs are simple pills. Inhalers, topical creams, injectables, and transdermal patches are harder to match. The active ingredient might be the same, but how it’s delivered can change everything. A cream’s texture, a puff’s particle size, a patch’s adhesive-these all affect how much drug gets into your body. That’s why the FDA has been working on new guidance since 2023. They’re developing better methods to test bioequivalence for these complex products. In November 2023, they released a draft for topical corticosteroids. In 2027, they’ll have new tools to ensure even these tricky drugs meet the same safety bar. They’re also partnering with MIT on a $2.3 million project to use artificial intelligence to predict which generic formulations might cause issues before they even hit the market. The goal? Catch problems early, before patients are affected.
What this means for you
If you’re on a long-term medication, especially one with a narrow therapeutic index, talk to your pharmacist. Ask if your generic has an ‘AB’ rating in the Orange Book. Don’t assume. Verify. If you notice a change in how you feel after switching-fatigue, dizziness, new side effects-don’t brush it off. Tell your doctor. But also ask: Was this a true therapeutic equivalence switch? Or did they swap you for a different drug altogether? The system works. It’s been tested, validated, and used for decades. Over 90% of prescriptions in the U.S. are now generics. And the savings? Nearly $1.7 trillion since 2009. But the real win isn’t the money. It’s the safety. You get the same treatment. At a lower cost. With no compromise.Frequently Asked Questions
Are generic drugs really as safe as brand-name drugs?
Yes-if they have an ‘AB’ rating in the FDA’s Orange Book. These generics must prove they deliver the same amount of active ingredient at the same rate as the brand. They’re tested in clinical trials and monitored for side effects. The FDA requires the same manufacturing standards for both. Over 90% of prescriptions in the U.S. are generics, and studies consistently show no difference in safety or effectiveness when substitution is based on therapeutic equivalence.
What does the ‘AB’ rating mean in the Orange Book?
The ‘A’ means the drug is considered therapeutically equivalent to the brand. The ‘B’ means it’s not. The second letter gives more detail-for example, ‘AB’ means the generic is bioequivalent and can be substituted. ‘AP’ means it’s an injectable with special packaging, but still equivalent. ‘BX’ means there’s insufficient data to confirm equivalence. Always check the code before accepting a substitution.
Can I trust a generic for a drug like warfarin or levothyroxine?
Yes, but with extra care. For drugs with a narrow therapeutic index, the FDA applies tighter bioequivalence standards-90-110% instead of 80-125%. Many patients switch safely between brands and generics. But because even small changes can affect outcomes, some doctors prefer to stick with one version. If you’re switching, your doctor may monitor your blood levels more closely at first. That’s not because generics are unsafe-it’s because precision matters more in these cases.
Why do some people say generics don’t work for them?
Often, it’s not the drug-it’s the change. Switching from a familiar brand to a different-looking generic can trigger anxiety, which sometimes feels like a physical side effect. Other times, patients are switched to a different drug entirely, not a true generic. Or the substitution involves a ‘B’-rated product, which shouldn’t have been swapped. Rarely, a manufacturing issue occurs. But when the generic has an ‘AB’ rating, the evidence overwhelmingly supports that it works the same.
How do I check if my generic is therapeutically equivalent?
Ask your pharmacist for the Orange Book rating. You can also search the FDA’s online Orange Book database for free. Just enter the brand name or generic name, and it will show the therapeutic equivalence code. If it says ‘AB,’ it’s safe to substitute. If it says ‘B’ or ‘BX,’ ask your doctor before switching. Don’t rely on appearance-color, shape, or packaging don’t indicate equivalence.





Ollie Newland
Man, I’ve been on levothyroxine for years and switched generics twice-no issues. The FDA’s Orange Book is legit. If it says AB, you’re good. People freak out over pill color, not science.
Martyn Stuart
Let’s be precise: therapeutic equivalence isn’t just bioequivalence-it’s bioequivalence + clinical outcome consistency. The FDA doesn’t just rely on AUC and Cmax; they require replicated studies across populations. And for narrow-therapeutic-index drugs like warfarin? They tighten the range to 90–110%. That’s not marketing-that’s rigorous pharmacology.
Also, the 2022 UnitedHealthcare survey of 12,500 patients? 87% reported no difference. That’s not anecdotal-it’s population-level data. When people say generics don’t work, they’re often conflating therapeutic interchange with substitution. Big difference.
And yes, the Orange Book is searchable. Type in your drug. Check the code. AB? You’re fine. B? Don’t switch. BX? Ask your pharmacist why it’s even on the shelf.
Stop letting fear drive decisions. The system works. It’s been validated for decades. And yes, it saves billions-but that’s not the point. The point is: you get the same medicine, at a fraction of the cost, without compromising safety.
Pharmacists are trained to do this. Trust them. Don’t assume. Verify.
Jessica Baydowicz
OMG YES!! I used to be super nervous about generics until my pharmacist sat me down and showed me the Orange Book codes-AB = all good!! Now I ask for them every time and save like $40 a month!! 🙌 Also, my mom’s on warfarin and switched without a hitch-her INR stayed perfect!!
George Graham
It’s wild how much trust we put into systems we don’t understand. The FDA’s Orange Book is one of those quiet, unsung heroes of public health. No fanfare, no headlines-just decades of data, peer-reviewed studies, and strict standards keeping millions safe.
I’ve worked with patients on phenytoin and levothyroxine for years. Some are terrified of switching. I get it. Change is scary. But when we walk them through the bioequivalence data-how the 80–125% range is statistically rigorous, how the FDA requires multiple crossover trials-it usually calms them down.
The real issue? Not the generics. It’s misinformation. Or worse-marketing from brand companies that profit from fear. I’ve seen it. A patient gets switched, feels a little off, and blames the generic. But when we check the record? They were switched to a different drug entirely. Not a generic. A different active ingredient.
Therapeutic equivalence isn’t a loophole. It’s a safeguard.
John Filby
Just checked my prescription-AB rating. Phew. I used to think generics were just cheap knockoffs until I read up on this. The fact that the FDA tests absorption rates in healthy volunteers and then confirms clinical outcomes? That’s next-level rigor. Also, the AI project with MIT? Super cool. Imagine predicting bad generics before they even hit shelves. Science is wild.
Ben Choy
My uncle had a seizure after switching to a generic. Scared the hell out of us. Turned out it wasn’t an AB-rated drug-it was a B-rated one. Pharmacist didn’t catch it. So yeah, check the code. Don’t assume. Always ask. And if your doc pushes back? Ask why. The Orange Book is public for a reason.
Emmanuel Peter
Let’s be real-this whole ‘therapeutic equivalence’ thing is a corporate scam. The FDA’s ‘data’? Mostly industry-funded. Bioequivalence studies are done in 20-30 healthy young men. What about elderly patients with kidney issues? Or people on 5 other meds? They don’t test that. And don’t get me started on the ‘AB’ rating-some generics have the same active ingredient but different fillers that cause reactions. You think the FDA cares? Nah. They’re in bed with Big Pharma. You’re a guinea pig.
Ashley Elliott
I appreciate the depth here. But I also think it’s worth noting: even with AB ratings, some patients do report changes. It’s rare, but it happens. And when it does, it’s not always about the drug-it’s about the psychological shift. The pill looks different. Feels different. That alone can trigger nocebo effects. So maybe the real takeaway isn’t just ‘trust the code’-but also, ‘trust your body, and speak up if something feels off.’
Chad Handy
Okay, so let me get this straight-you’re telling me that after 15 years of taking the same brand-name drug, I’m supposed to just switch to some random generic because the FDA says so? And you think I’m just gonna sit there and not feel anything different? I’ve had panic attacks, insomnia, brain fog-everything. I don’t care what the Orange Book says. My body knows. And I’m not some lab rat for corporate cost-cutting. They’re not testing for long-term effects. They’re not testing for interactions with my antidepressants. They’re not testing for how it affects my sleep cycle. And yet, somehow, we’re supposed to trust a 90% confidence interval from a study done on 25 college kids? That’s not science. That’s negligence. And now I’m stuck with this weird, chalky pill that tastes like regret. I’ve been on this drug for 15 years. I don’t want to change. I don’t want to be a statistic. I want my medicine to feel like it always did. And if the system won’t let me have that, then the system is broken.
And don’t even get me started on the fact that the generic I got last month was a different color than the one before. Same box. Same name. Different pill. That’s not equivalence. That’s chaos.
They say ‘90% of people don’t notice a difference.’ But what about the 10%? Who’s listening to us? Nobody. Because we’re not profitable. We’re just patients.
Augusta Barlow
They told me the same thing about vaccines. And now look. The FDA’s ‘Orange Book’? It’s a front. I did my own research. There’s a whole underground network of pharmacists who know that the bioequivalence tests are rigged. The 80-125% range? That’s a joke. A 25% swing in absorption could mean life or death for someone on warfarin. And they’re letting pharmacies swap these out automatically? No doctor approval? No consent? This is how people die quietly. And no one talks about it because the media’s bought off. I’ve got a friend who had a stroke after switching. They blamed her age. But I know better. It was the generic. The FDA doesn’t want you to know this. That’s why they bury it in jargon. ‘Therapeutic equivalence’? Sounds fancy. But it’s just corporate speak for ‘we don’t care if you die as long as we save a buck.’
Scott van Haastrecht
So you’re telling me that a pill that costs $2 instead of $200 is ‘exactly the same’? That’s not science-that’s delusion. You think the fillers, the binders, the coating don’t matter? They do. And when you’re on a narrow-therapeutic-index drug, even a 2% difference in dissolution rate can trigger a seizure. I’ve seen it. I’ve filed reports. The FDA ignores them. They don’t want to admit that generics can fail. Because then they’d have to stop the automatic substitution. And that would cost Big Pharma money. So they let people suffer in silence. And you? You’re just another sheep reading their press release.
And don’t even get me started on the AI project with MIT. That’s just PR. They’re not fixing the system-they’re trying to make you feel better about it. Meanwhile, the real problem? No one’s tracking long-term outcomes. No one’s comparing generics across batches. No one’s auditing manufacturing. It’s a black box. And you’re okay with that? Pathetic.
Shofner Lehto
Just want to clarify one thing: therapeutic equivalence doesn’t mean identical. It means clinically equivalent. That’s the difference. Two drugs can have different inactive ingredients and still be AB-rated. The FDA doesn’t require identical fillers-only identical clinical outcomes. That’s why you sometimes get a different-colored pill. Doesn’t mean it’s different. Just different packaging. Don’t let appearance scare you. Check the code. That’s all you need.