Every year, millions of Americans fill prescriptions for generic drugs-cheaper versions of brand-name medicines that work the same way. Nine out of ten prescriptions in the U.S. are for generics. They save the system over $300 billion annually. But behind those low prices is a fragile system. Too many companies chasing tiny profits. Too few willing to make the hard-to-produce drugs. And when one factory shuts down, the whole supply can collapse.
The illusion of choice
It looks like there’s plenty of competition in the generic drug market. Dozens of companies sell versions of common pills: metformin for diabetes, lisinopril for blood pressure, amoxicillin for infections. But appearances are misleading. For most of these drugs, only one or two manufacturers actually keep them in stock. Take epinephrine auto-injectors, the life-saving devices for allergic reactions. In 2023, a major U.S. plant producing generic versions shut down due to FDA violations. Within weeks, hospitals ran out. Patients had to switch to brand-name EpiPens-costing up to 10 times more. Why? Because only three companies made generic versions. One quit production. The other two couldn’t ramp up fast enough. This isn’t rare. In 2024, IQVIA found that 35% of generic drug markets had fewer than three active manufacturers. Twelve percent had just one. These aren’t obscure drugs. They’re the ones people rely on daily: antibiotics, heart meds, insulin, seizure controls. When competition disappears, so does supply.Why do manufacturers leave?
The problem starts with price. Generic drugs are supposed to be cheap. And they are-sometimes too cheap. After a brand-name drug loses patent protection, the first few companies to enter the market make decent profits. But then others rush in. Prices plunge. Within three years, the average price drops by 20%. By year five, it’s often down 80% from the original brand price. For simple pills, that’s fine. Manufacturing costs are low. But for complex drugs-like sterile injectables, inhalers, or biosimilars-the costs are high. Building a clean-room facility for injectables can cost $500 million. It takes two years to get FDA approval. Then you need to run it 24/7 with trained staff, constant testing, and strict documentation. If you’re selling a generic antibiotic for 5 cents a pill, and your production cost is 4 cents, you’re barely breaking even. One recall. One FDA warning letter. One delay in raw material shipment-and you’re in the red. So companies quit. They shift resources to more profitable products. Or they exit the U.S. market entirely. That’s why the top five manufacturers control nearly half the sterile injectable market. They’re the only ones who can afford to play. Everyone else got priced out.The FDA’s double-edged sword
The FDA wants more competition. So it approved 956 generic applications in 2023-90 of them first-time generics. That’s good for patients. More options. Lower prices. But the agency also cracked down harder on quality. In 2023, the FDA issued 147 warning letters for data fraud, poor sanitation, or fake test results in generic drug plants. That’s a 23% jump from 2022. Many of these violations came from plants in India and China-the same places that supply most of the world’s generic drugs. When the FDA shuts down a plant, demand doesn’t vanish. It floods to the remaining factories. But those factories are already running at 90% capacity. They can’t suddenly make more. So shortages happen. And they happen fast. The FDA’s Drug Competition Action Plan was meant to speed up approvals. It did. But it didn’t fix the underlying problem: too many companies entering, too few staying. And when the only suppliers are barely profitable, one hiccup can break the chain.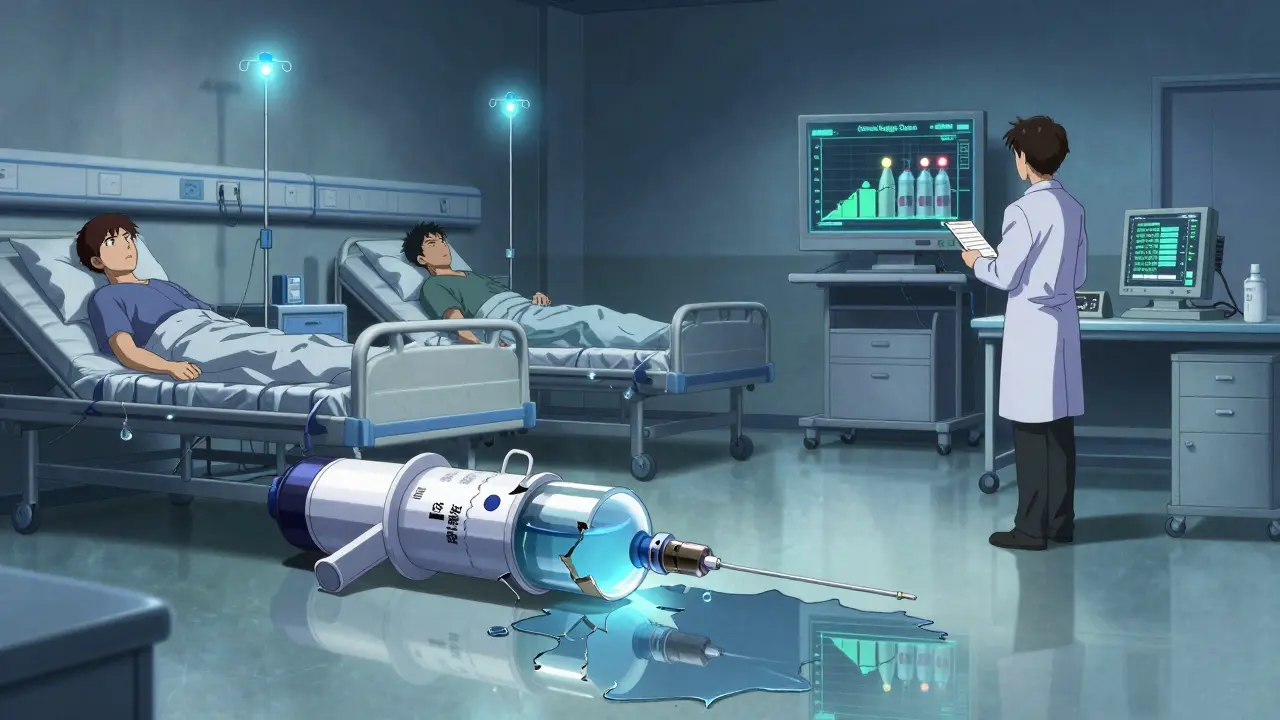
Who pays the price?
Doctors see it every day. A 2023 survey by the American Medical Association found 78% of physicians dealt with at least one generic drug shortage in the past year. Nearly half said these shortages frequently disrupted care. Cardiovascular drugs. Antibiotics. Chemotherapy agents. These are the most affected. A patient with high blood pressure might get switched from one generic lisinopril to another. But if none are available, they’re stuck with the brand name-costing $150 a month instead of $5. That’s a 30x difference. Many patients skip doses. Or stop taking it altogether. Hospitals scramble. They use older, less effective alternatives. Or they buy from distributors who charge triple the normal price. Insurance companies hate it. Patients hate it. But the manufacturers? They’re already gone. Meanwhile, the biggest generic drugmakers-Teva, Sandoz, Aurobindo, Sun Pharma-are making billions. But they don’t make the cheap, low-margin drugs anymore. They focus on high-demand, complex products. Or they buy up smaller competitors to control more of the market. Consolidation isn’t helping. It’s making shortages worse.The 4-to-6 rule
The European Medicines Agency studied this exact problem in 2024. Their conclusion? For essential medicines, you need 4 to 6 manufacturers. Not one. Not two. Four to six. Why? Because if one plant fails, the others can pick up the slack. If prices drop too low, the market still has enough profit to keep everyone in business. And if a new company wants to enter, there’s enough volume to make it worth their while. Right now, only 65% of essential generic drugs in the U.S. and Europe meet that standard. The rest? One or two suppliers. High risk. Low resilience. Some countries have solved this. Canada and the U.K. use centralized purchasing. They guarantee minimum volumes to manufacturers. That gives companies confidence to invest in production. The U.S. doesn’t do that. It lets the market decide. And the market chooses profit over reliability.





Ayodeji Williams
Bro, this is wild 😅. I got my insulin from a 3-cent generic last month, now it's $12 and they say 'out of stock'. Meanwhile, my cousin in India gets it for 2 cents and the factory's still running. Who's really being screwed here? 🤡
Christine Joy Chicano
The structural flaw isn't just price erosion-it's the absence of strategic buffer. The U.S. treats essential pharmaceuticals like commodities, not public infrastructure. You don't let the market dictate the supply of life-sustaining medicines the way you do toothpaste. The FDA's approval frenzy without supply-chain safeguards is like opening a thousand fast-food joints and then blaming them when one runs out of buns.
There’s zero incentive to maintain redundant capacity when you’re competing on a 0.5% margin. We need to decouple production viability from retail price volatility. That means guaranteed volume contracts, not just more paperwork.
Adam Gainski
This is spot on. I work in hospital pharmacy and we’ve had to switch patients 3x in 8 months just to find a version of metformin that’s actually in stock. One time we had to use a 1980s formulation because the modern one was unavailable. Patients get confused, side effects spike, and no one takes responsibility. It’s a mess.
And yeah, the big players like Teva aren’t making the cheap stuff anymore-they’re hoarding the profitable ones. We need to treat generics like public utilities. Maybe even nationalize production for critical drugs.
Sai Ganesh
India produces over 60% of the world’s generic drugs. The FDA’s crackdown on Indian plants is not about quality-it’s protectionism disguised as safety. The same standards are ignored in U.S. and EU facilities. Why do you think the price of amoxicillin doubled after the FDA shut down five Indian plants? Because they replaced them with U.S. manufacturers who charge 4x more.
We need global harmonization, not isolation. If the FDA wants better quality, audit and certify. Don’t blockade.
Emma Addison Thomas
It’s fascinating how we celebrate market efficiency until it fails us. We’ve outsourced the production of our most vital medicines to distant factories, then act surprised when supply chains break. The U.S. doesn’t just lack policy-it lacks a moral framework for healthcare infrastructure. Profit can’t be the only metric for survival.
Jonathan Larson
One must consider the philosophical underpinnings of this crisis: the conflation of economic efficiency with social utility. The market, left unregulated, optimizes for marginal cost reduction, not systemic resilience. The consequence is not merely economic-it is existential. When a diabetic patient cannot access insulin because the lowest-bidder factory was shut down for noncompliance, the market has failed not as an allocator, but as a moral agent.
What we require is not more competition, but reconstituted responsibility. The state must become the guarantor of essential pharmaceutical redundancy-not as a monopolist, but as a steward. The FDA’s role must evolve from gatekeeper to architect of sustainable supply.
Canada’s centralized procurement model is not socialist-it is rational. It recognizes that some goods, by virtue of their necessity, cannot be subject to the whims of speculative pricing. To deny this is to deny the very premise of public health.
And yet, we cling to the myth of the invisible hand, as if it could mend a broken vial or refill an empty syringe. The hand is invisible because it has long since abandoned the sick.
Aparna karwande
Of course the U.S. has shortages-because you let your country’s medicine be made by foreign labs that don’t even follow basic hygiene! We in India make 80% of your generics, and your FDA treats us like criminals. Meanwhile, your own plants? Silent. Your politicians? Silent. But when a child can’t get epinephrine? Suddenly you scream about ‘American jobs’?
Wake up. This isn’t about competition. It’s about hypocrisy. You want cheap drugs? Fine. But don’t blame the makers for your own greed. We make them. You just don’t want to pay the real cost.
Stop pretending you care about patients. You care about your quarterly reports. And your patients? They’re collateral.
Anastasia Novak
OMG. I just realized-I’ve been taking lisinopril for 5 years and never knew it was made in a factory that could be shut down by a single broken thermometer. 😭
Like, how is this even legal? My blood pressure is literally dependent on the whim of some guy in Hyderabad who didn’t wash his hands. This is a horror movie. I’m calling my senator. And also, someone please send me a 3-month supply of EpiPen before I die.
Elen Pihlap
Why don’t they just make more? It’s not that hard to make pills. I mean, I can bake cookies and they don’t even need FDA approval. Why can’t they just… make more? 😩
Andrew N
Actually, the real issue is that the FDA doesn’t have enough inspectors. It’s not about competition or price. It’s just underfunding. If they had 500 more inspectors, they could approve more plants and avoid these shortages. Simple fix. No policy changes needed.
Paul Mason
Yeah but if you look at the data, the real problem is that nobody wants to make the boring drugs anymore. I mean, who’s gonna invest $500 million to make 5-cent antibiotics? That’s just dumb. The market’s doing what it’s supposed to-shifting to where the money is. You can’t blame capitalism for being capitalist.
Just pay more. Or die. It’s that simple.